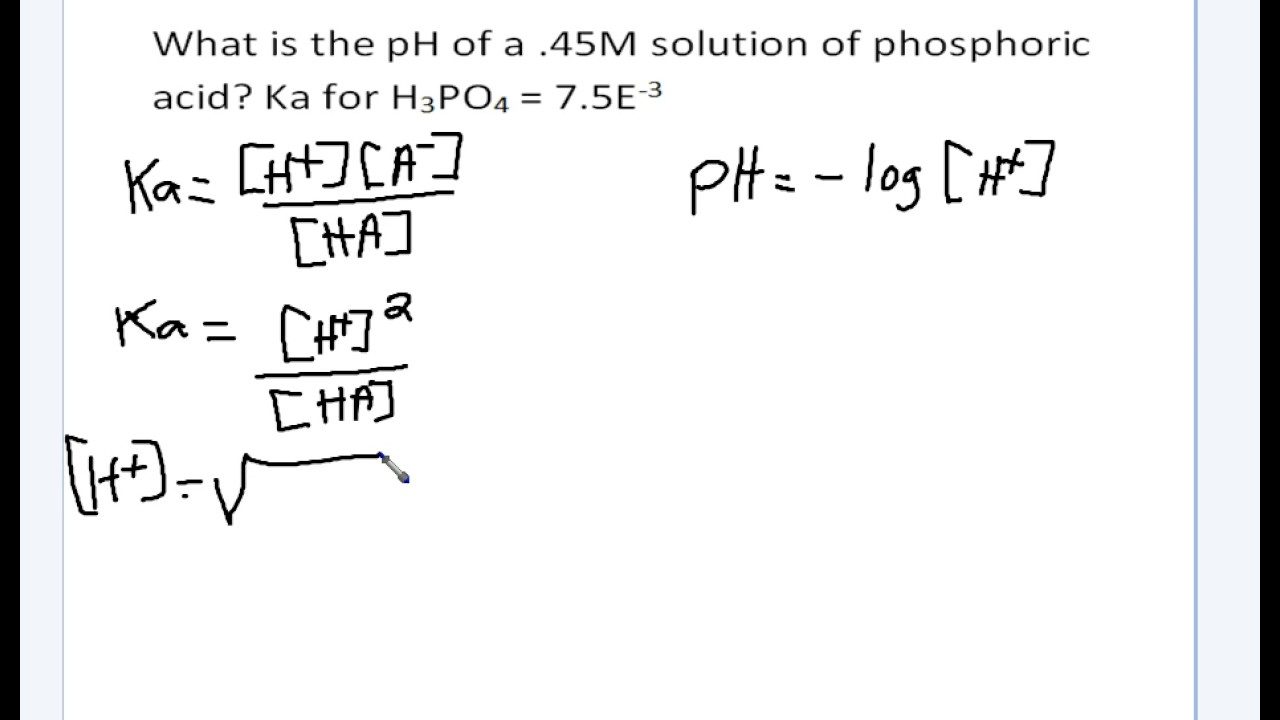
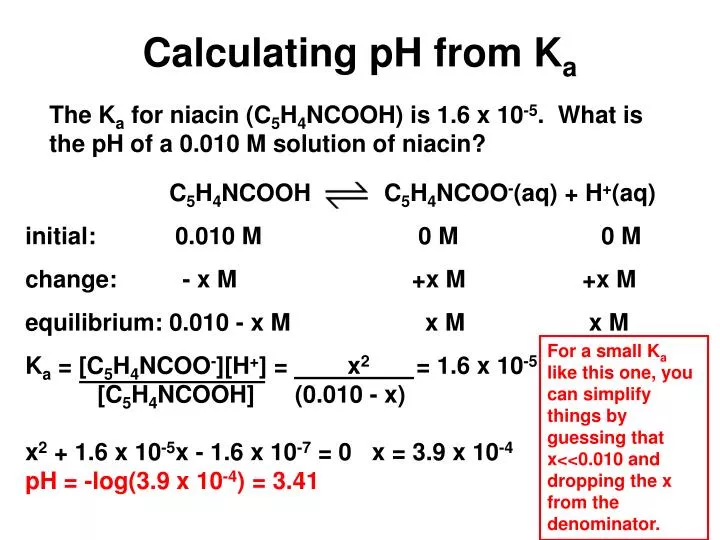
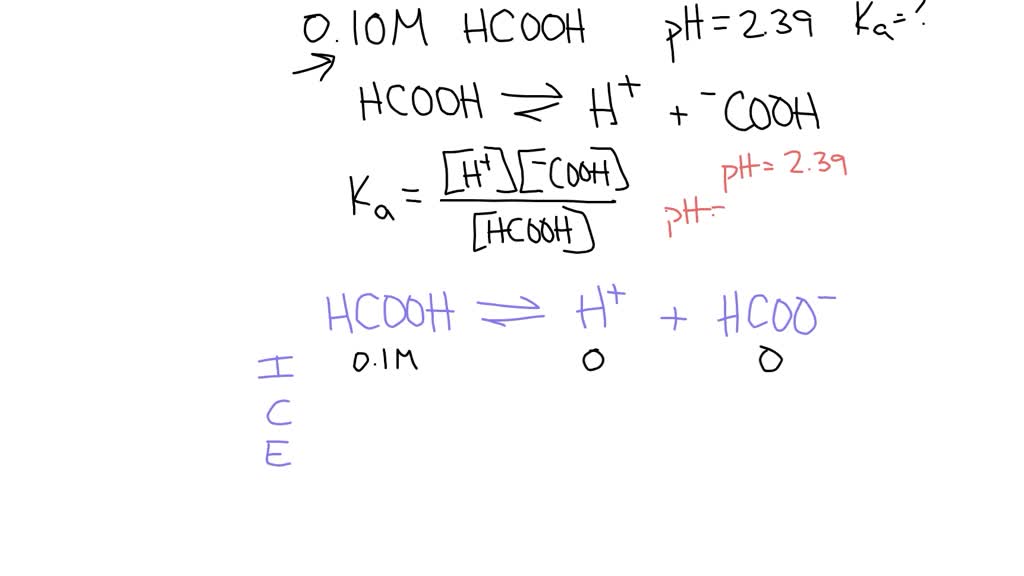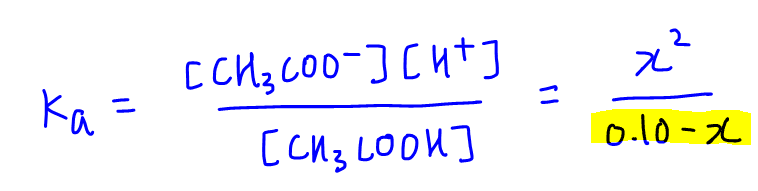How to Calculate Ka (Acid Dissociation Constant) of a Weak Acid Shortcut, Problems, and Examples - YouTube
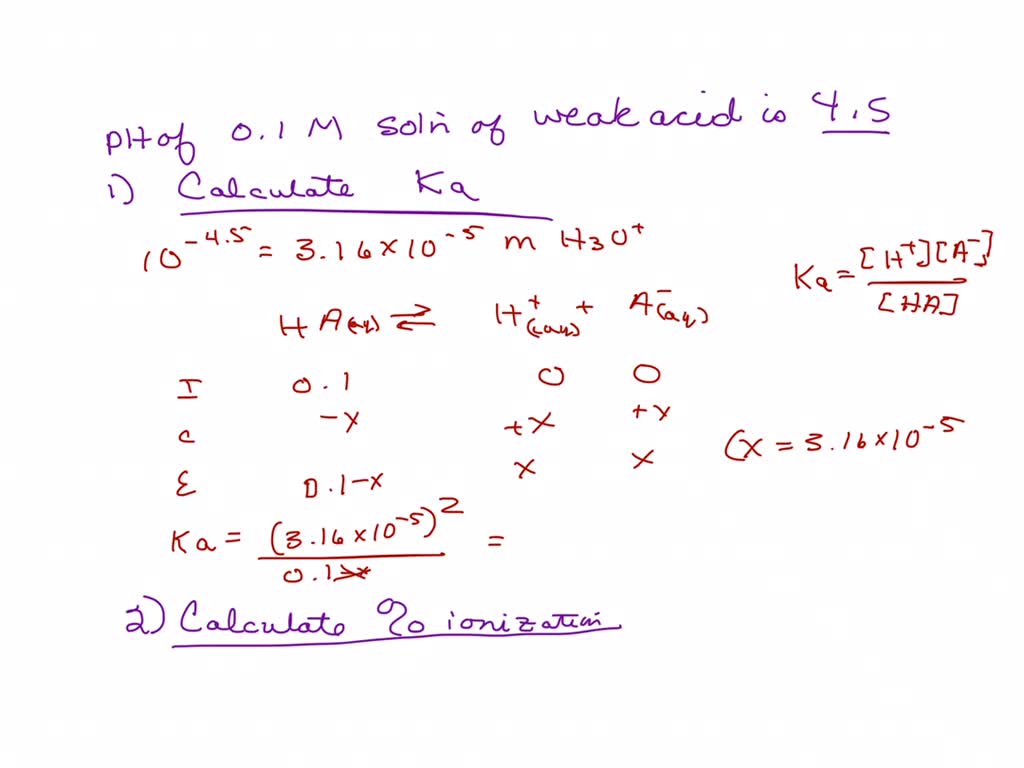
SOLVED: CHEM 1312 NAME The pH of a 0.1 M solution of the weak acid HA is 4.5. Calculate the Ka for the acid. (5 POINTS) b. Calculate the percent ionization for the acid. (3 POINTS)
A 0.15 m solution of chloroacetic acid has a pH of 1.86. What is the value of Ka for this acid? - Quora